How To Draw An Amino Acid
26.one Structures of Amino Acids
- Page ID
- 91056
Objectives
After completing this section, you should be able to
- identify the structural features present in the twenty amino acids commonly constitute in proteins.
Note: Yous are not expected to remember the detailed structures of all these amino acids, but yous should be prepared to draw the structures of the 2 simplest members, glycine and alanine.
- draw the Fischer projection formula of a specified enantiomer of a given amino acid.
Note: To do and so, y'all must remember that in the Due south enantiomer, the carboxyl grouping appears at the top of the projection formula and the amino group is on the left.
- classify an amino acid equally being acidic, basic or neutral, given its Kekulé, condensed or autograph structure.
- depict the zwitterion form of a given amino acid.
- account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation.
- write advisable equations to illustrate the amphoteric nature of amino acids.
Cardinal Terms
Make certain that you lot can define, and utilise in context, the key terms below.
- α‑amino acids
- amphoteric
- essential amino acids
- zwitterion
Study Notes
This is a good point at which to review some of the principles of stereochemistry presented in Chapter 5. Be sure to brand total use of molecular models when any stereochemical issues arise.
You should recognize that a three‑alphabetic character autograph code is ofttimes used to stand for private amino acids. You demand not memorize this code.
The distinction betwixt essential and nonessential amino acids is not as articulate‑cutting as one might suppose. For instance, arginine is oftentimes regarded every bit being nonessential.
Introduction to Amino Acids
Amino acids class polymers through a nucleophilic attack by the amino group of an amino acid at the electrophilic carbonyl carbon of the carboxyl group of another amino acid. The carboxyl group of the amino acid must first exist activated to provide a better leaving group than OH-. (We volition discuss this activation by ATP later in the course.) The resulting link between the amino acids is an amide link which biochemists call a peptide bond. In this reaction, h2o is released. In a contrary reaction, the peptide bail can be cleaved past water (hydrolysis).
- Structure and Belongings of the Naturally-Occurring Amino Acids (Likewise large to include in text: impress separately)
When two amino acids link together to course an amide link, the resulting structure is called a dipeptide. Likewise, we can have tripeptides, tetrapeptides, and other polypeptides. At some signal, when the structure is long plenty, information technology is called a protein. At that place are many different ways to represent the structure of a polypeptide or protein, each showing differing amounts of information.
Figure: Different Representations of a Polypeptide (Heptapeptide)
Figure: Amino Acids React to Form Proteins
(Note: above picture represents the amino acid in an unlikely protonation land with the weak acid protonated and the weak base of operations deprotonated for simplicity in showing removal of water on peptide bond germination and the hydrolysis reaction.) Proteins are polymers of twenty naturally occurring amino acids. In contrast, nucleic acids are polymers of but iv different monomeric nucleotides. Both the sequence of a protein and its full length differentiate 1 protein from some other. Merely for an octapeptide, at that place are over 25 billion different possible arrangements of amino acids. Compare this to just 65536 different oligonucleotides of viii monomeric units (8mer). Hence the diversity of possible proteins is enormous.
Stereochemistry
The amino acids are all chiral, with the exception of glycine, whose side concatenation is H. As with lipids, biochemists utilize the L and D nomenclature. All naturally occuring proteins from all living organisms consist of L amino acids. The absolute stereochemistry is related to L-glyceraldehyde, as was the case for triacylglycerides and phospholipids. Most naturally occurring chiral amino acids are S, with the exception of cysteine. As the diagram below shows, the absolute configuration of the amino acids can exist shown with the H pointed to the rear, the COOH groups pointing out to the left, the R group to the right, and the NH3 group upwards. You tin can remember this with the anagram CORN.
Effigy: Stereochemistry of Amino Acids.
Why do biochemists still apply D and Fifty for sugars and amino acids? This explanation (taken from the link below) seems reasonable.
"In addition, all the same, chemists often need to ascertain a configuration unambiguously in the absence of any reference chemical compound, and for this purpose the alternative (R,S) system is ideal, as it uses priority rules to specify configurations. These rules sometimes lead to absurd results when they are applied to biochemical molecules. For example, equally we have seen, all of the common amino acids are L, considering they all have exactly the aforementioned construction, including the position of the R grouping if nosotros but write the R group as R. However, they exercise non all accept the same configuration in the (R,South) system: L-cysteine is also (R)-cysteine, simply all the other L-amino acids are (Due south), but this merely reflects the human decision to give a sulphur atom higher priority than a carbon atom, and does not reflect a existent difference in configuration. Worse problems can sometimes arise in commutation reactions: sometimes inversion of configuration can result in no change in the (R) or (S) prefix; and sometimes retention of configuration can result in a change of prefix.
It follows that information technology is not simply conservatism or failure to sympathize the (R,Due south) system that causes biochemists to continue with D and Fifty: it is merely that the DL arrangement fulfils their needs much improve. Equally mentioned, chemists also utilize D and L when they are advisable to their needs. The explanation given above of why the (R,S) system is footling used in biochemistry is thus well-nigh the verbal opposite of reality. This arrangement is actually the but practical way of unambiguously representing the stereochemistry of complicated molecules with several disproportionate centres, but it is inconvenient with regular series of molecules like amino acids and simple sugars. "
Natural α-Amino Acids
Hydrolysis of proteins by boiling aqueous acid or base of operations yields an array of small molecules identified equally α-aminocarboxylic acids. More than twenty such components have been isolated, and the nearly common of these are listed in the following table. Those amino acids having green colored names are essential nutrition components, since they are non synthesized by human metabolic processes. The best nutrient source of these nutrients is protein, but information technology is of import to recognize that not all proteins have equal nutritional value. For example, peanuts have a higher weight content of protein than fish or eggs, only the proportion of essential amino acids in peanut protein is but a 3rd of that from the 2 other sources. For reasons that will get evident when discussing the structures of proteins and peptides, each amino acid is assigned a one or iii letter abbreviation.
Natural α-Amino Acids
Some common features of these amino acids should be noted. With the exception of proline, they are all 1º-amines; and with the exception of glycine, they are all chiral. The configurations of the chiral amino acids are the same when written as a Fischer projection formula, as in the drawing on the correct, and this was defined every bit the L-configuration past Fischer. The R-substituent in this structure is the remaining structural component that varies from 1 amino acid to another, and in proline R is a iii-carbon chain that joins the nitrogen to the alpha-carbon in a 5-membered ring. Applying the Cahn-Ingold-Prelog annotation, all these natural chiral amino acids, with the exception of cysteine, take an S-configuration. For the start seven compounds in the left column the R-substituent is a hydrocarbon. The last 3 entries in the left cavalcade have hydroxyl functional groups, and the commencement two amino acids in the right column comprise thiol and sulfide groups respectively. Lysine and arginine have basic amine functions in their side-chains; histidine and tryptophan have less bones nitrogen heterocyclic rings as substituents. Finally, carboxylic acid side-chains are substituents on aspartic and glutamic acrid, and the last 2 compounds in the correct column are their corresponding amides.
The formulas for the amino acids written above are unproblematic covalent bond representations based upon previous understanding of mono-functional analogs. The formulas are in fact incorrect. This is evident from a comparing of the concrete properties listed in the post-obit table. All four compounds in the table are roughly the same size, and all have moderate to excellent water solubility. The starting time two are simple carboxylic acids, and the 3rd is an amino booze. All three compounds are soluble in organic solvents (e.1000. ether) and accept relatively low melting points. The carboxylic acids have pKa'south near 4.five, and the conjugate acrid of the amine has a pKa of ten. The unproblematic amino acid alanine is the last entry. By contrast, it is very loftier melting (with decomposition), insoluble in organic solvents, and a 1000000 times weaker equally an acid than ordinary carboxylic acids.
Physical Backdrop of Selected Acids and Amines
| Chemical compound | Formula | Mol.Wt. | Solubility in Water | Solubility in Ether | Melting Point | pKa |
|---|---|---|---|---|---|---|
| isobutyric acid | (CHiii)2CHCOtwoH | 88 | 20g/100mL | consummate | -47 ºC | v.0 |
| lactic acrid | CH3CH(OH)CO2H | 90 | consummate | complete | 53 ºC | 3.9 |
| 3-amino-two-butanol | CH3CH(NH2)CH(OH)CH3 | 89 | complete | complete | 9 ºC | 10.0 |
| alanine | CHthreeCH(NHii)CO2H | 89 | 18g/100mL | insoluble | ca. 300 ºC | ix.8 |
Zwitterion
These differences above all point to internal salt formation by a proton transfer from the acidic carboxyl function to the basic amino grouping. The resulting ammonium carboxylate structure, commonly referred to every bit a zwitterion, is also supported by the spectroscopic characteristics of alanine.
| CHthreeCH(NH2)COtwoH |  | CH3CH(NH3) (+) COii (–) |
As expected from its ionic character, the alanine zwitterion is loftier melting, insoluble in nonpolar solvents and has the acid forcefulness of a 1º-ammonium ion. Examples of a few specific amino acids may also be viewed in their favored neutral zwitterionic course. Notation that in lysine the amine office uttermost from the carboxyl grouping is more basic than the alpha-amine. Consequently, the positively charged ammonium moiety formed at the chain terminus is attracted to the negative carboxylate, resulting in a coiled conformation.
The structure of an amino acid allows information technology to human activity as both an acid and a base of operations. An amino acid has this power considering at a certain pH value (dissimilar for each amino acrid) well-nigh all the amino acid molecules be as zwitterions. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acrid becomes positively charged. If base of operations is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid. In both circumstances, the amino acid acts to maintain the pH of the system—that is, to remove the added acid (H+) or base (OH−) from solution.
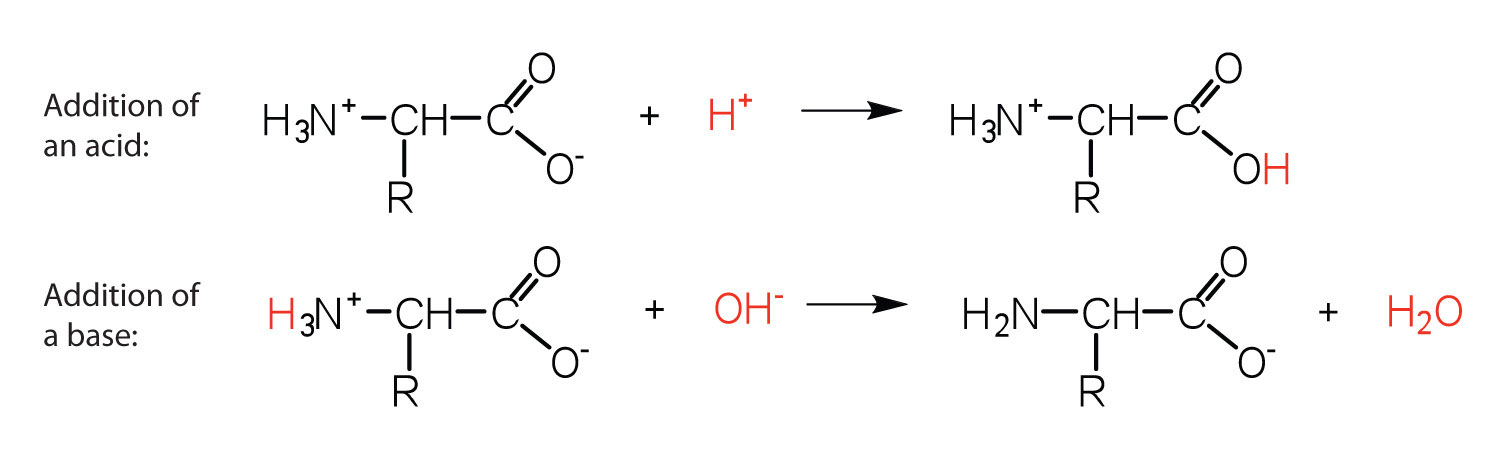
Example 26.ane
- Draw the structure for the anion formed when glycine (at neutral pH) reacts with a base.
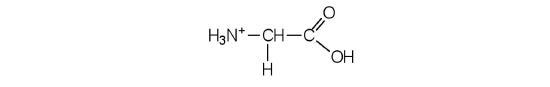
- Draw the structure for the cation formed when glycine (at neutral pH) reacts with an acid.
Solution
-
The base removes H+ from the protonated amine grouping.
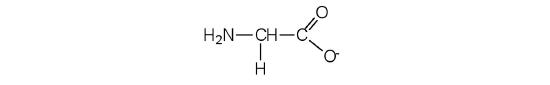
-
The acid adds H+ to the carboxylate grouping.
Other Natural Amino Acids
The twenty alpha-amino acids listed above are the primary components of proteins, their incorporation beingness governed by the genetic lawmaking. Many other naturally occurring amino acids exist, and the structures of a few of these are displayed below. Some, such every bit hydroxylysine and hydroxyproline, are but functionalized derivatives of a previously described compound. These two amino acids are institute just in collagen, a common structural poly peptide. Homoserine and homocysteine are higher homologs of their namesakes. The amino group in beta-alanine has moved to the end of the three-carbon chain. It is a component of pantothenic acid, HOCHtwoC(CH3)2CH(OH)CONHCHtwoCHtwoCO2H, a member of the vitamin B complex and an essential nutrient. Acetyl coenzyme A is a pyrophosphorylated derivative of a pantothenic acid amide. The gamma-amino homolog GABA is a neurotransmitter inhibitor and antihypertensive amanuensis.
Many unusual amino acids, including D-enantiomers of some common acids, are produced past microorganisms. These include ornithine, which is a component of the antibiotic bacitracin A, and statin, plant every bit role of a pentapeptide that inhibits the action of the digestive enzyme pepsin.
Exercises
Questions
Q26.1.i
Why is cysteine the only L amino acrid with an R configuration at the alpha carbon?
Q26.1.2
Isoleucine has two stereogenic centers.
(a) Draw a Fischer projection of isoleucine.
(b) Draw a Fischer projection of an isoleucine diastereomer, and label each stereocenter as R or Southward.
Solutions
S26.1.1
The sulfur atom in the side chain causes the side chain to have higher priority than the other substituents.
S26.i.2
(a)

(b)


Source: https://chem.libretexts.org/Courses/Athabasca_University/Chemistry_360%3A_Organic_Chemistry_II/Chapter_26%3A_Biomolecules%3A_Amino_Acids_Peptides_and_Proteins/26.01_Structures_of_Amino_Acids
Posted by: listergioncy.blogspot.com


0 Response to "How To Draw An Amino Acid"
Post a Comment